Despite advances in replacements for animal models in medical testing, we have yet to find a comprehensive and viable replacement for animal models. Until that time comes, modern research will continue to be driven by established ethical principles, such as the 3Rs and the 5 Freedoms. The minimization of animal discomfort and suffering is of utmost importance, achieving this also provides researchers with generalizable data. Failing to do so not only causes unnecessary suffering, it negates the purpose of the research as the animal’s capacity to be models for humans would be undermined by negative health status.

The collection of accurate and generalizable data is contingent on happy and healthy test animals, thus any efforts to enrich the lives of laboratory animals is essential to effective research. The 3Rs, Reduction, Replacement & Refinement, is an initiative in line with this goal.
Briefly summarized, the goal of 3Rs is to reduce the suffering of animals by decreasing the number of animals used in research, replacing animals with alternatives wherever possible, and refining current research practices to further reduce suffering. The initiative of the 3R’s has contributed significant positive change in the lives of laboratory animals and has improved the quality of collected data. Refinements like social housing for rats & dogs appreciably improved their overall health and quality of life. Replacements such as the use of in-vitro human cells for migration & proliferation assays have helped reduce the number of animals required for research.
Some study designs however, present issues that require creativity and ingenuity to overcome. When working with dogs, exercise is an important form enrichment, both in terms of social development during play with other dogs, and in terms of releasing excess energy. Exercise is very simple with the overwhelming majority of study designs and test item administration methods. Continuous infusion studies however, present unique challenges that need to be overcome.
In this newsletter, we discuss the importance of exercise as a form of enrichment, issues associated with continuous infusion studies with dogs, and how ITR has created a novel solution to allow for exercise where it was previously not possible.

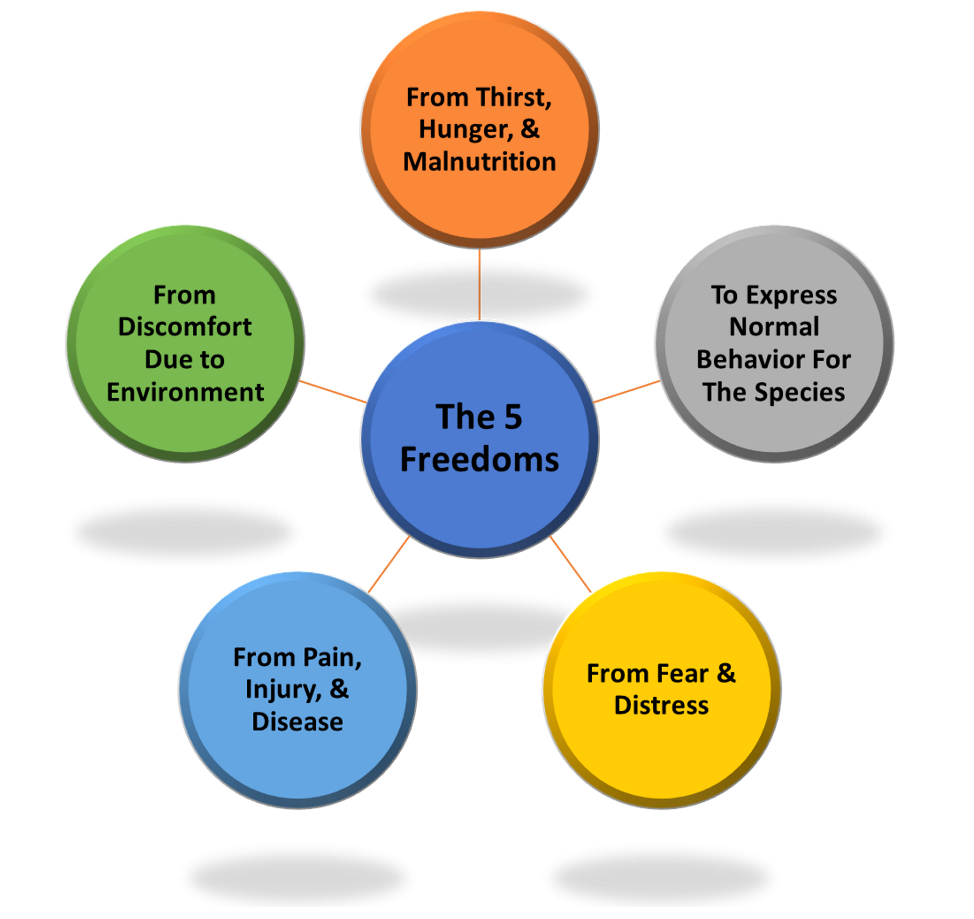
In order for humans to conduct ethical research with animals and in order to provide humans with the best possible model for testing, the animals must be as physically and mentally healthy as possible. Modern ethical principles in animal research date back to 1965 England, from a report regarding animal welfare by the UK government. From this report, the concept of the 5 Freedoms were conceptualized. The 5 Freedoms are: freedom from hunger & thirst, freedom from discomfort, freedom from pain, injury or disease, freedom to express normal behavior and freedom from fear & distress.
These freedoms, initially conceptualized for farm animals & livestock, were also implemented in animal research. Standardized modern laboratory procedures refined by organizations such as the CCAC and the AAALAC account for each of these 5 freedoms. Laboratory animals are adequately fed and have free access to water, social species are housed together with comfortable bedding, a dedicated veterinary staff ensures the physical and mental health of the animals, and animals are given adjustment time to adapt to their new surroundings while being slowly introduced to laboratory equipment to minimize distress.
Dogs, like humans, are a social species that love to play and need exercise to maintain a healthy body and mind. Thus, it is important to allow exercise for dogs in order to meet the freedom to express normal behavior. With most study designs, dogs are easily exercised, however, continuous infusion studies need special considerations.

Standard procedure for continuous infusion studies requires general anesthesia and surgical implantation of a catheter connected to a jacket & tether system to enable test item administration via an infusion pump. The catheter remains implanted for the duration of the study. While the test item is not being administered, saline solution is administered to prevent backflow of blood, introduction of bacteria or other complications with the fluid flow to the catheter. In order to enable exercise, researchers have had two options: remove the catheter from the animal, or unplug from the infusion pump. Both of these options present complications.
Removing and replacing the catheter would require repeated anesthesia and surgery, which adds unnecessary stress as well as requiring technician time and surgical supplies. Most continuous infusion studies are conducted over a period of up to 28 days, on rare occasions, they can last for a period of up to 9 months. To make room for weekly exercise, surgery would need to be performed multiple times per week for the duration of the study. Repeated removal and implantation of catheters compromises the health and wellbeing of the animal, presents high risk of infection, puts unnecessary strain on the veterinary staff and creates noise in the research data. Removing the catheter to enable exercise is thus not a viable solution.
The other alternative to enable exercise requires disconnection directly from the pump. However, the tubing from the pump to the animal needs to be long enough to ensure the animal can comfortably move in their housing cages while maintaining connection to the infusion pumps and the saline reservoirs on the outside of the housing cages. The length of the tube connected to the animal complicates the standard rough & tumble playstyle of dogs. The long tubing needs to be held by technicians to ensure that the connection to the animal remains intact and isn’t disturbed during exercise. This severely limits the forms of exercise the animals are capable of engaging in and does not allow the dogs to express their natural behaviors.

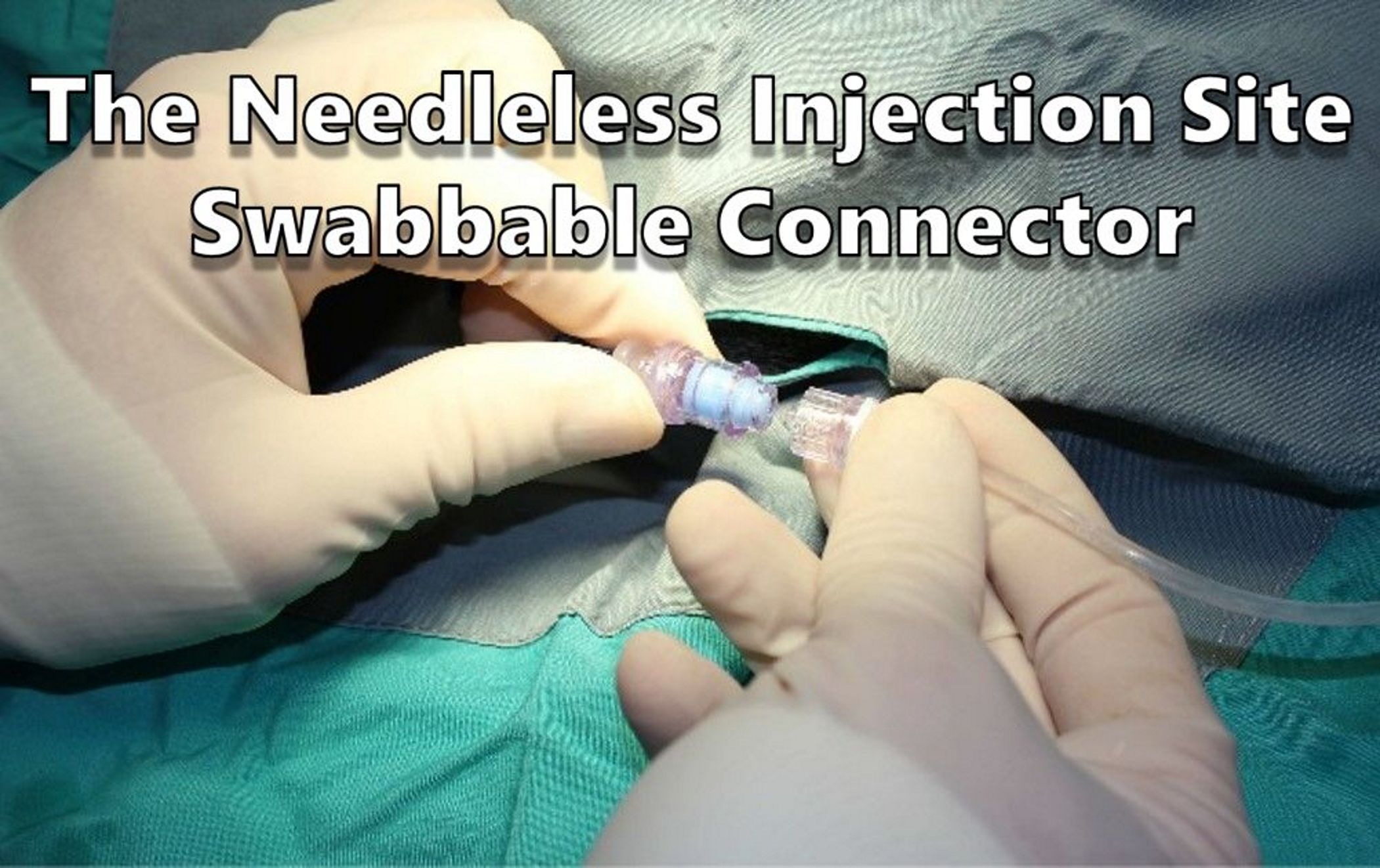
To overcome the long tubing problem, the catheter was fitted with a needleless injection site swabbable connector (NISSC), which serves as a secondary connection point between the catheter and the infusion pump. The NISSC is located near the dorsal catheter exit site (Fig. 1) and is covered with a specially made jacket with a flap to secure the connector during exercise.
While disconnected, the NISSC is capped with a disinfecting cap for needleless connectors (Fig. 2) to prevent the introduction of bacteria into the fluid path, this helps prevent infection. During exercise, the dogs could now be disconnected from the pump without excess tubing because the connector can be secured under the flap of the jacket.
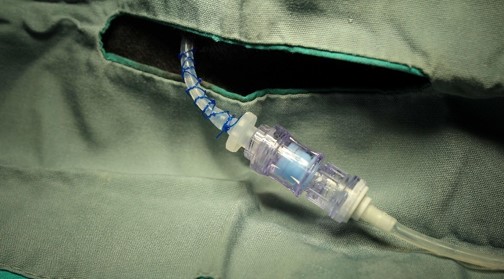
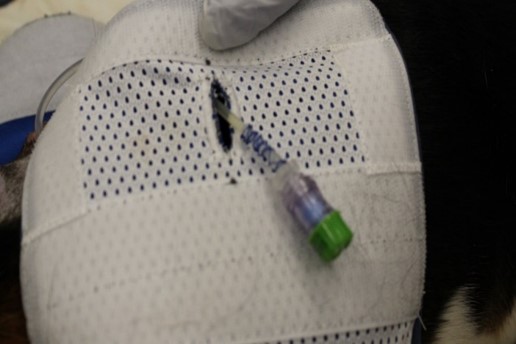
The NISSC allows the dogs to freely engage in their natural play behaviors without risk of catheter displacement and without damage or tangling of the tubing during exercise. Disconnection and reconnection take very little time and the disinfecting cap also wards against infection. The dogs can then be easily reconnected to the pump to resume dosing after exercise.

To determine the viability of the NISSC as a solution to allow for exercise during continuous infusion studies, four dogs were fitted with an NISSC and were infused with sodium chloride over 28 days. Two weeks after surgery, they were disconnected from the pump at the level of the NISSC and allowed 20 minutes of exercise 5 times per week for two weeks for a total of 10 sessions of exercise.
At the end of the experiment, no negative changes in body weight, food consumption or behavior were discovered and no signs of infection were found. The NISSC was considered to be a safe, viable and very practical tool in permitting dog exercise on continuous infusion studies.
Until medical science advances to the point where we have created complete replacements for animals in research, ethical research practices will remain in priority. ITR will continue to innovate and create simple yet elegant solutions such as the NISSC to improve the lives of research animals.