Safe and successful drug development requires a thorough understanding of how a new drug will move through the body (Pharmacokinetics) and how it will interact with the body (Pharmacodynamics). Among the battery of tests that new drugs undergo, Receptor Occupancy is a rapidly developing measure of pharmacodynamics that has become more prevalent in the age of personalized therapies. Through the use of Flow Cytometry, Receptor Occupancy (RO) data provides researchers with insight into how a drug binds to its target receptors in the body.
With RO data from human cells, non-human primates or other laboratory animal species and additional supporting preclinical PD/PK data, researchers can predict how the human body will respond to the drug and they can make informed dosage decisions. Moving to human trials without appropriate understanding of the drug receptor binding events can lead to disastrous consequences, causing permanent damage and even death in human volunteers.
In this newsletter, we will discuss Flow Cytometry, Receptor Occupancy and how it is measured, as well as the importance of Receptor Occupancy data in preclinical drug development research.
Flow Cytometry (Fig 1.) is an analytical technique used to differentiate and discriminate between multiple different physical or chemical properties of cells by passing cells single file through a laser. The light from the laser will scatter differently based on the physical and chemical properties of the cell. The light scatter is recorded by sensors and rendered into graphical data by a computer. Filtering the cells one at a time allows for researchers to differentiate between many different cells within a heterogenous cellular population.
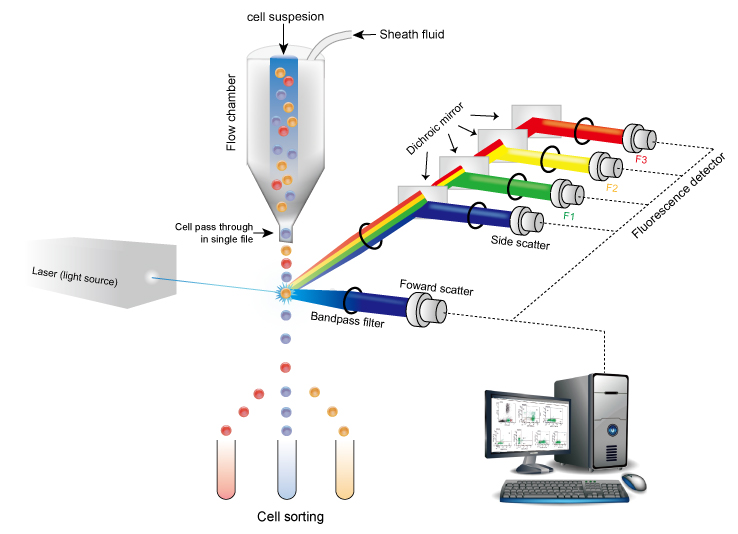
Fig 1. Flow Cytometry
Additionally, cells can be fluorescently tagged, which will create unique light scatter when passing through the laser corresponding to the fluorescent tag used. Using this technique, researchers can employ fluorescently conjugated antibodies that bind to specific cellular target receptors to gain insight into receptor activity for drug development.
Receptor Occupancy (RO) is a measure of the binding of a biotherapeutic to its cellular target. Receptor Occupancy data can be used to answer a variety of questions related to the pharmacodynamics of a new drug. Which cell populations do the drug bind to? Which receptors do the drug bind to? Does the drug cause upregulation or downregulation of the receptor? How much of the available receptors on the cellular target are occupied by the drug?
RO data along with pharmacokinetic data can help researchers determine safe dosage levels for First-In-Human trials without unexpected toxicity or life threatening side effects.
The development of TGN1412 in 2006 in Europe provides an example of how RO data can be used to mitigate potential disaster when moving from preclinical to clinical trials. During development of the immunomodulatory drug TGN1412, established procedures determined a No Adverse Effects Level in cynomolgus monkeys. However, due to biological differences between C028 receptor biology in monkeys vs humans, the NOAEL led to a dosage level roughly 25 times too high for humans. As a result, the first clinical trial led to a life threatening cytokine storm in the 6 volunteers.
Review of the data in the case of TGN1412 revealed that the use of ex-vivo and in-vivo RO assessments along with additional biomarker testing could have prevented the nearly lethal mistake.
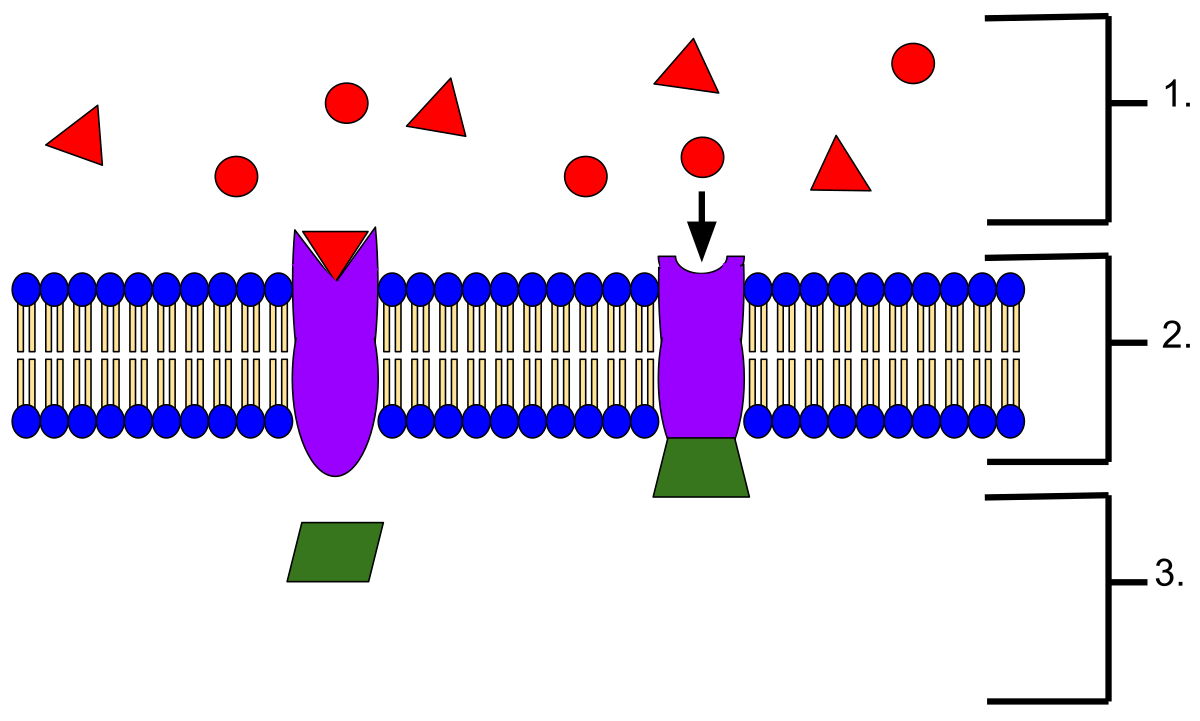
Receptor Occupancy is measured most often using fresh whole blood specimens. There are three common methods for measuring Receptor Occupancy, researchers will employ a combination of these methods: Drug Free Receptor, Drug Occupied Receptor, and Total Receptor.
The Drug Free Receptor method (Fig 2.) measures the proportion of receptors not bound by the drug. This is accomplished by fluorescently tagging the drug itself, a competitive antibody or the receptor ligand.
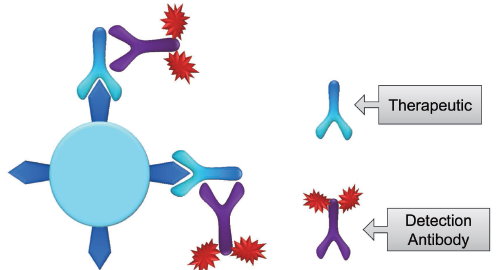
Fig 2. Free Receptor Assay Using Conjugated Drug or Competing Antibody
The Drug Occupied Receptor method (Fig 3.) measures the proportion of drug occupied receptors by introducing a fluorescently tagged antidrug that does not compete with the drug binding receptor. This antidrug will bind to the drug itself after the drug has bound to the receptor, allowing us to determine how many receptors have been bound by the drug.

Fig 3. Bound Receptor Assay Using Anti Therapeutic Antibody
The Total Receptor method (Fig 4.) measures both free and bound receptors by using a fluorescently tagged non-competing antibody. This tagged antibody will provide an accurate representation of the total available receptors.
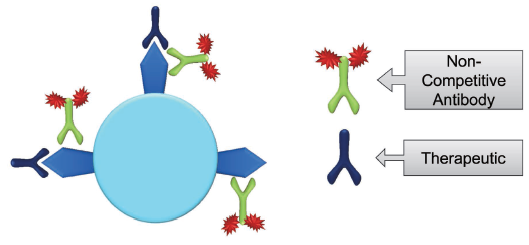
Fig 4. Total Receptor Assay Using Non-Competitive Antibody
The nature of Receptor Occupancy assay research necessitates development and validation of a new assay for each study drug and each assay presents new challenges to overcome. As development of personalized cancer treatments continue to progress, the need for Receptor Occupancy data will continue to grow.
Assay development and validation has numerous roadblocks and confounds. Beyond reagent conflicts and sample stability problems researchers must also consider the biological differences between subjects. Each test subject will be affected differently by the drug. Subjects will generate different amounts of anti-drug-antibodies (ADA), and the drug itself can also modulate the cell counts differently in each subject. These issues can affect the binding of the drug to the target receptors or simply reduce the available number of receptors, both of which have implications for the three measurement methods discussed earlier. As a result, post-dose data need to be normalized against baseline data.
Successful assay development requires trained and experienced scientists. Equipped with two BD FACSCanto II Flow Cytometers, ITR’s expert immunology team is well prepared to help with your Receptor Occupancy research.
