
As the global medical and scientific community mobilizes to find a solution to the global pandemic caused by the novel coronavirus, many different options are being explored. Vaccines are an effective way of stopping communicable diseases from spreading. However, vaccine development is a lengthy process, often taking from 2 to 5 years before they are ready for the public. Today’s novel coronavirus is not the first coronavirus in recent history. SARS-CoV-1 in 2003 and MERS-CoV in 2012 were also coronaviruses and although they had a much higher case-fatality rate, they were far less contagious. Upon the discovery of SARS-CoV-1, work began swiftly on a vaccine. It took scientists 4 months before the genome sequence was available to develop antigens that could be used for in vitro and in vivo animal trials. It wasn’t until December of 2004 when the first human trials commenced, but the disease was well contained by that time and the development of the vaccine was ultimately discontinued.
Medical sciences have greatly evolved since 2003 and SARS-CoV-2 had been sequenced by early January 2020. Many companies have vaccine candidates currently in preclinical trials and some have candidates already in human trials. Unlike the coronaviruses from 2003 and 2012, SARS-CoV-2 is far more contagious and containment is no longer an option so it is unlikely that vaccine development will halt before completion in its case.
Vaccines will help keep healthy people from getting sick, but those with compromised immune systems or certain underlying health conditions cannot be vaccinated and vaccination cannot cure those who are already sick. To protect the widest range of people, developing therapeutics along with vaccines is necessary. Among the current therapeutic options currently in development are antibody therapeutics. These treatments are a form of passive immunization as opposed to the active immunization conferred by vaccination or natural exposure.
In this newsletter we will discuss active and passive immunization, antibody therapeutics and key considerations for their preclinical evaluation, and we will present ITR’s experience with safety testing for antibody therapeutics.

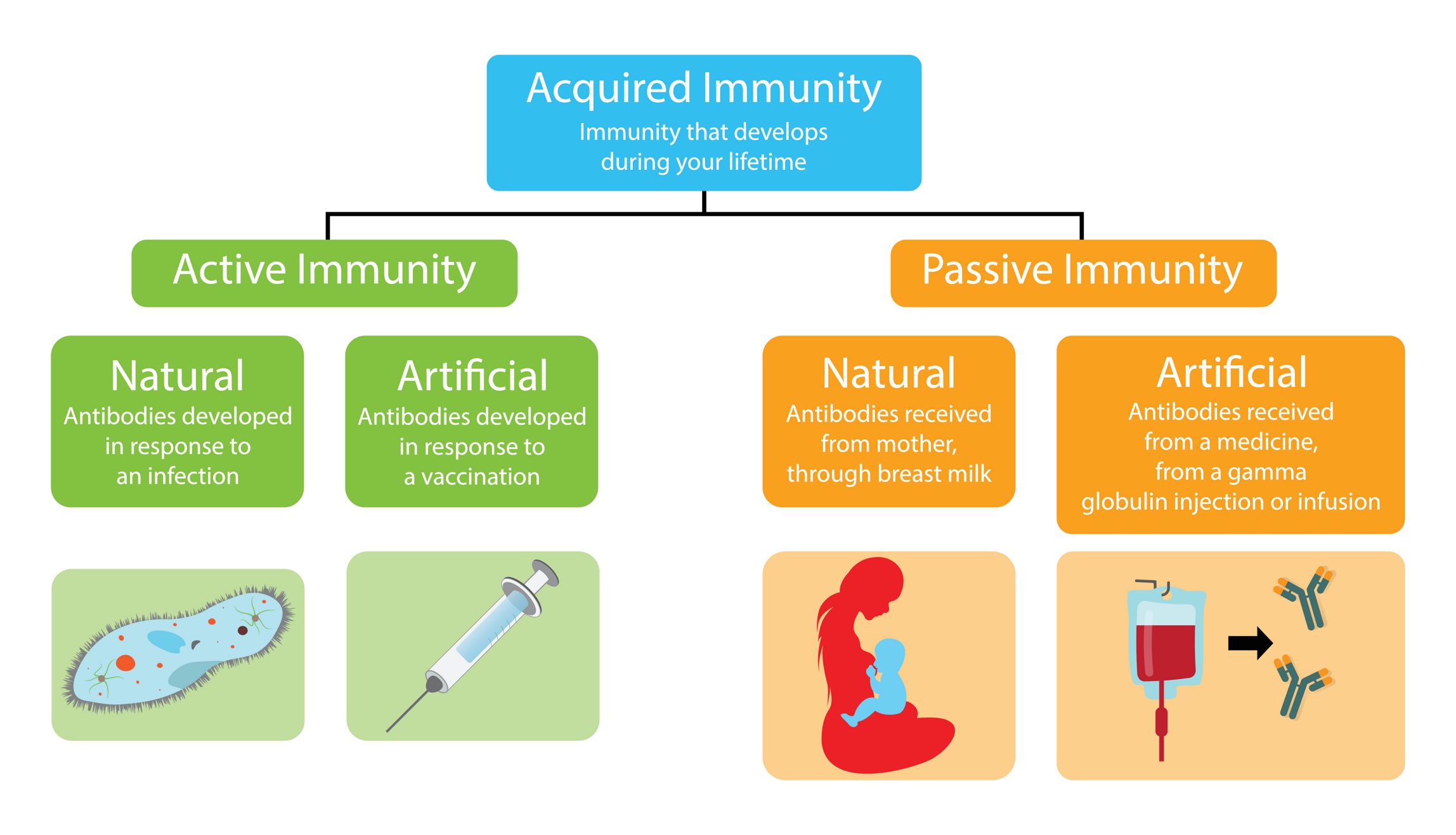
Active immunity is the result of antibody production by the body following exposure to foreign molecules. Antibodies are produced by the B lymphocytes following the activation of the adaptative immune response and their production can be voluntarily stimulated against a specific target through vaccination using weakened versions of the pathogen of interest. Actively acquired immunity typically provides long lasting protection because of immunological memory which causes a quicker and more powerful immune response when antigenically-similar pathogens are detected by the body in future. exposures. While active immunity does provide long term protection, it is impractical in the case of highly toxic substances or for pathogens with short incubation periods due to the delay between the introduction of the antigen and the production of antibodies.
Antibodies can also be acquired from external sources to provide passive immunity. Infants receive maternal antibodies through breastfeeding and antibodies are transferred to the fetus in utero. Antibodies can be taken from those who have recovered from infectious disease and injected into those who are currently infected to help fight the infection. Artificial antibodies can be engineered and injected therapeutically as well. However, the duration of protection offered by passive immunization is shorter than active immunity because the immune system does not learn to produce the necessary antibodies as effectively and future infection with antigenically similar pathogens is not going to produce a sufficiently powerful immune response.

Antibodies have been used as therapeutics in medicine since the late 18th century. Botulism, diphtheria and tetanus were all treated with therapeutic antibodies. These antibodies were produced by injecting animals with an antigen to induce an immune response in the animal. Blood is then drawn and the antibody-rich serum is injected into human patients. Antibodies found in serum are known as polyclonal antibodies (pAbs) because they are produced from multiple clonal populations of B cells and they are capable of binding to multiple different epitopes of a single antigen.
At the time, this process was called serum therapy. Horse antisera showed some successes with treating toxin-related diseases as well as some viruses such as polio and influenza. While animal-produced serum showed some positive therapeutic effects, there were however numerous challenges related to animal immunization, batch to batch variability of serum and adverse side effects in humans.
Human plasma and immunoglobulins from convalescent or immunized humans came into use around the 1930s and are still used today to treat children with antibody deficiencies. Although generally safer than animal serum, human-derived antibody therapies still posed the risk of transmitting infections like Hepatitis B and C. Serum therapy fell out of favor after the emergence of antibiotics and sulfonamides for the treatment of most bacterial infections in the 1930s to 1940s.
The development of hybridoma technology in 1975 allowed for the mass production of identical antibodies referred to as monoclonal antibodies (mAbs), which demonstrate a high affinity for a single epitope on the target. This technology eliminated much of the burden associated with the production and use of animal or human-derived pAbs. The first generations of mAbs saw limited clinical successes because of their animal origins which resulted in poor interaction with human biology. Today, mAbs are designed with entirely human frameworks to reduce immunogenicity caused from animal-derived mAbs. These antibodies are used today to treat a wide variety of inflammatory diseases such as rheumatoid arthritis and various cancers, as well as viral infections like influenza. Antibody therapies can also be used to offer a short-term protection to those who are unable to be vaccinated.
Despite the advantages, even humanized mAbs present safety concerns and complications that require careful preclinical evaluation.

Monoclonal antibodies have eclipsed the use of polyclonal antibodies due to more reliable manufacturing processes, better characterization of the mode of action, better safety profiles and capacity to treat a wider range of human diseases. As with other forms of medicine, preclinical trials are intended to provide all the information necessary to perform safe human trials; determining a safe starting dose, identifying which organs are likely to be targeted for toxicity, identifying the parameters for safety monitoring in humans and whether any observed toxicities are reversible.
As such, parameters often measured as part of the non-clinical safety evaluation study package include, analysis of the test item concentration in the dose formulations, clinical observations, body weight and food consumption measurements, ophthalmological assessments, evaluations of the potential effect of the mAbs on the cardiovascular, respiratory and central nervous system, as well as clinical pathology (hematology, coagulation, clinical chemistry and urinalysis) evaluations. A series of blood samples is also collected on the first and last day of dosing at selected time points relative to treatment and used for the analysis of test item concentrations in plasma/serum and the subsequent calculation of toxicokinetic parameters. Additional blood samples are also often collected at selected time points relative to treatment, and depending on the target and mechanism of action of the antibody, used for immunophenotyping, analysis of cytokine levels, T cell-dependent antibody response (TDAR), the presence of anti-drug antibodies and/or other biomarkers.
Gaining regulatory approval to test new drug products in humans requires testing in two relevant animal species, however as with other biologic drugs, mAbs are often not pharmacologically active in most laboratory animal species, as such performing the non-clinical safety program in one relevant species is often sufficient to satisfy regulatory requirements.

ITR’s expert immunology team is trained and equipped to perform all the required preclinical testing for large molecule drugs, including mAbs, in both large and small animals. ITR offers a wide range of in-vitro GLP assay types and formats, including but not limited to: dose formulation analysis by absorbance and fluorescence, bioanalysis by ELISA or ECL, as well as GLP and non-GLP biomarker assays used to evaluate pharmacodynamics and organ toxicities.
Other in-vitro and in-vivo GLP assays more specific to antibodies include: standard rat and monkey T cell-dependent antibody response (TDAR) assays, natural killer cells activity assay, immunophenotyping, receptor occupancy and multiplex cytokine assays by Meso Scale Discovery (MSD) or cytometric bead arrays. ITR can also perform GLP validation or non-GLP qualification of a new biomarker on demand. For a complete list of currently available biomarkers, click Here.
Our flexible team can support any advanced assay requests and method development is always performed to meet GLP standards even for non-GLP projects.

As the race for a vaccine for the novel coronavirus continues to be the center of public attention, therapeutic treatments are being developed in parallel. While vaccines are a powerful tool in the fight against infectious disease, they are not a catch-all solution. In order for them to be effective, vaccines must be administered prior to virus exposure. Solving this crisis will require both prophylactic treatments in the form of vaccines to halt the spread of the virus and therapeutic medicines to help save the lives of those whose condition does not allow for vaccination to be an option. Antibody treatments are versatile in their applications by serving as therapeutics for a wide variety of indications, such as inflammatory diseases cancers and viral infections, as well as prophylactics by providing short term passive immunity for those who are unvaccinated.
ITR is equipped and ready to help manage this crisis by providing comprehensive safety profiles for treatments, whether they be vaccines or antibody therapeutics.
